AeroTime is excited to welcome Dr. Klaus Radermacher KRBE GmbH as our columnist. With over 35 years in management, science, and consulting, Dr. Radermacher brings a wealth of experience in analyzing and assessing transportation systems. His innovative approach integrates infrastructure and movement processes into holistic CO2 and energy comparisons.
Follow Dr. Klaus Radermacher’s LinkedIn newsletter “Think. Mobility. Differently.” for regular insights on mobility concepts and transportation systems.
The views and opinions expressed in this column are solely those of the author and do not necessarily reflect the official policy or position of AeroTime.
There is hardly any other topic in discussions about future mobility and its energy that has raised so many eyebrows as Sustainable Aviation Fuel (SAF), sometimes generally referred to as “electricity-based synthetic fuels.”. For some, these fuels, which are ideally CO2-neutral, are the future with which combustion engines can continue to be operated without any problems. Others see them as niche products with no major future impact, which are at most suitable candidates for “greenwashing”. In such discussions, which are often emotional and not necessarily fact-based, each party usually has one or two examples at hand to support their respective position. For example, the first transatlantic flight with SAF, which, as a singular event, certainly demonstrated technical possibilities, but can by no means be seen as a turning point towards CO2-neutral aviation.
In this article, I will provide an overview of some of the relevant backgrounds, technologies, and developments in this context.
As in all my articles, I focus on scientific contexts as well as facts, figures, and data, leaving ideologies and emotions aside. Given the very large number of existing projects, prototypes, funding measures, plans for large-scale plants, etc., it goes without saying that this can only be a current snapshot with no claim to completeness.
A little bit of basic chemistry (Organic Chemistry 101)
In order to understand the discussions and the significance of SAF, it is necessary to familiarize yourself with a few very basic chemical relationships.
All fuels (petrol, diesel, kerosene, etc.) are hydrocarbons, which differ only slightly chemically and consist of carbon atoms and hydrogen atoms that have formed bonds in different molecules. The differences between hydrocarbons lie in the number of atoms in each molecule.
The simplest molecule of this type is methane (gas), which makes up by far the largest proportion of natural gas. A methane molecule consists of one carbon atom to which four hydrogen atoms are bonded, hence the chemical formula CH4. If you have two carbon atoms, each of which has a bond with the second carbon atom, you still need a total of six hydrogen atoms to make a stable molecule. C2H6 is ethane, also a gas. With three C atoms and then eight H atoms, you have propane (C3H8), also a gas that is contained under pressure and thus liquefied in propane gas cylinders used for barbecuing with gas or for radiant heaters in outdoor catering. The chains of these hydrocarbons can be much longer; there can be four, eight, 10, 15 or more carbon atoms connected in a row within a molecule, each of which then has an H atom on all the free bonds. A molecule always requires “twice as many H atoms plus two” as there are carbon atoms, so the “calculation of the sum formula” is correspondingly simple. Butane (gas) is C4H10, pentane C5H12, hexane C6H14, etc. Octane, which we know as the “octane rating” for a criterion of gasoline quality, is C8H18.
The first four hydrocarbons (methane, ethane, propane, butane) are gaseous under “normal conditions” (20 degrees C, 1013 hPa), from pentane upwards they are liquid. All hydrocarbons are combustible and burn together with oxygen to form carbon dioxide (CO2) and water (H2O), which evaporates due to the combustion temperatures. For example, if we consider two molecules with 12 C and 26 H atoms each, the “reaction equation” of complete combustion is as follows:
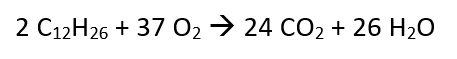
Two molecules of C12H26 burn with 37 molecules of oxygen (O2) to form 24 molecules of carbon dioxide (CO2) and 26 molecules of water (H2O). Those interested in chemistry can reproduce the corresponding stoichiometric calculations at any time. Two moles of C12H26 weigh 340 g and burn with 37 moles of O2 (1,184 g) to form a total of 1,056 g of CO2 and 468 g of water. The weights of the substances involved in the chemical reaction are the same before and after combustion; nothing is lost in such a process.
Properties of kerosene and other hydrocarbons
All fuels consist of a mixture of different hydrocarbons. The molecules are not always the aforementioned carbon chains; ring-shaped structures are also almost always found, and “branches” in the molecular structure and double bonds between carbon atoms are also possible. In addition, additives are added to ensure certain properties (temperature behavior, corrosion protection, etc.); fuels are therefore always standardized on the basis of their properties and not on the basis of their exact chemical composition.
SAFs are therefore also differentiated according to whether they are approved as “drop-in”, which means that they can be mixed with conventional kerosene and used in existing aircraft fleets at any time. The EU’s Renewable Energy Directive also specifies, among other things, which raw materials may be used for SAF.
The advantage of hydrocarbons for energy generation is the high amount of energy present in the substances, which is released during combustion and can be utilized. The widely used “Jet A1” kerosene contains an energy of 43.1 megajoules (MJ) per kilogram. Because it is also liquid under normal conditions, this amount of energy only requires a volume of around 1.2 liters, which corresponds to a volume-specific energy density of around 36 MJ per liter. Pure hydrogen, which is gaseous under normal conditions and must be stored in tanks under very high pressure for use as a fuel, only has an energy density of 4.8 MJ/l even at a pressure of 700 bar.
This advantage of high energy density is, at least for the time being, accompanied by the disadvantage that kerosene, gasoline, diesel, etc. are almost exclusively obtained from crude oil in refinery processes. Although the carbon contained in crude oil was originally removed from the Earth’s carbon cycle tens of millions of years ago, it has since been bound in the crude oil and is only released into the atmosphere as CO2 when the petrochemical products are burned.
What can SAFs be produced from?
SAF are hydrocarbons in which the carbon is not of fossil origin but comes from other sources. Ideally, it comes directly from the air and thus reduces the CO2 content in the atmosphere, or it is used directly from other CO2 sources, which then emit less CO2. The CO2 may also have been produced by photosynthesis over the last few months or years. In photosynthesis, the most important chemical process in nature, CO2 from the air is converted into biomass using water and light, i.e., solar energy, which binds the carbon; the oxygen bound in the CO2 is released into the environment.
However, the carbon can also be of a completely different origin, from waste fats from the food industry, waste from agriculture and forestry to feces, basically anything is possible. Some processes that have been developed in recent years are referred to by the following terms and abbreviations: HEFA (Hydro processed Esters and Fatty Acids) and HVO (Hydrotreated/Hydrogenated Vegetable Oils). Here, fats, which also always consist of the elements carbon, hydrogen, and oxygen (chem.: glycerol esters), are processed through a chemical process chain in such a way that hydrocarbons, among other things, are produced as the end product. From a chemical point of view, it does not matter whether it is used fats or fresh fats, such as palm oil, which is obtained from the fruit of the oil palm. In principle, there is nothing wrong with using new vegetable fat, as long as the conditions under which it is produced are appropriately taken into account. If, as a first step, virgin forests are destroyed by slash-and-burn to plant palm oil plantations, the CO2 balance will not be positive when viewed holistically.
Biogases obtained from organic waste and self-produced biomass can also be processed into SAF and e-fuels. Many of these production methods are based on a 100-year-old technique: the Fischer-Tropsch process (FT synthesis), originally developed in Germany to produce liquid fuels from domestic hard coal in order to be self-sufficient from the rest of the world. The process was also of great economic importance in the South African apartheid regime because South Africa had large quantities of coal, but no crude oil. For SAFs, the carbon in the FT process does not come from hard coal, but from biomass. Over the years, different variants of FT synthesis have been developed, which have been optimized with regard to the primary resources available. These processes have also been tested on an industrial scale.
For an overall CO2 balance, it is crucial for all processes for the production of synthetic hydrocarbons:
- which primary raw materials are used,
- which energy is used in the process,
- which transport routes are required for the starting materials and
- how other waste products can be used.
SAFs can also be produced from alcohols, which, from a chemical point of view, also only consist of carbon, hydrogen, and oxygen, and are sometimes referred to as AtJ (Alcohol-to-Jet). In this process, the biomass is first fermented into alcohol and then processed into SAF.
In the United States, there are now companies that grow fast-growing biomass, such as corn or millet, on huge areas that did not require deforestation in advance in order to produce SAF. Critics like to argue that this creates competition between food production and SAF in agriculture. However, this criticism is not always factually well-founded, as it overlooks the fact that, for example, when growing corn, only a tiny fraction of the plant, namely the maize kernels on the cob, are suitable as food for humans. The rest of the huge plants are by-products or waste products in terms of food and can therefore be available for SAF production. This applies equally to straw from grain production or waste cellulose. Viewed holistically, it is an excellent process chain: solar energy (light) drives photosynthesis, which removes CO2 from the atmosphere and adds oxygen. The carbon in the resulting biomass is transformed by chemical processes into hydrocarbons, which are used in combustion engines for propulsion and only emit the exact amount of CO2 that was previously removed from the atmosphere. This is CO2-neutral if the energy required to transform biomass into kerosene was also produced in a CO2-neutral way.
Outlook
In conclusion, it can be said that liquid hydrocarbons are well suited as propulsion energy for transportation systems that cover long distances and provide high transport performance due to their mass and volume-specific energy density. An aircraft carrying around 300 passengers achieves a transport performance of around 3,000,000 PKM (passenger kilometers) on a single 11 to 12-hour long-haul flight. Even a modern and efficient aircraft will burn 60 to 70 tons of kerosene and emit 190 to 220 tons of CO2 for such a route. However, 60 tons of kerosene also represent more than 2.5 million MJ of energy required for this transport performance and given the current state of technology, no other forms of energy or energy storage are expected in the medium term that could represent a realistic and economical alternative.
The development of largely or completely sustainable hydrocarbons that are as CO2-neutral as possible is still in its infancy in terms of the quantities required. In a press release from December 2022, the International Air Transport Association (IATA) estimates the demand for SAF for 2050 at 450 billion liters per year. In 2022, 300 million liters were available worldwide, an increase of more than 200% compared to 2021. For 2050, a 1,500-fold increase in production compared to 2022 would have to be achieved.
To express the sober 1,500-fold in a different way: If we take the IATA numbers as a basis, the entire amount of SAF available in 2022 will last for just under six hours in 2050 to refuel all aircraft with SAF everywhere and all year round.
Is this possible? The future is always difficult to predict, but we have another 25 years to get there. Looking at what happened in innovation fields over the last 25 years, I see no reason it should not be possible. But we had better hurry up! We will certainly not get to the required quantities by eating more French fries and hoping for enough used cooking oil, that is for sure!
With the theoretical foundations fully understood, it is mainly a question of large-scale engineering and appropriate incentives to make a technology commercially viable. Scientists, engineers, aviation managers and politicians must come up with a holistic and feasible concept to make this happen.
Another important issue for the time being is the price of SAF, especially in comparison to fossil kerosene. In 2019, the price of the “cheapest SAF” from the HEFA process was 1,200 $/t, roughly double the price of conventional kerosene ($600/t). In 2022, it was 1,100 $/t for conventional and more than 2,400 $/t for SAF. Due to the increasing demand, which is also the result of legal requirements for SAF blending, the prices for SAF are expected to rise and the price difference between that and conventional kerosene might increase even further.
The challenges to be overcome are enormous, but the essential foundations have been laid. In any case, considerable efforts will be required for decades to come in order to achieve the goals that have already been formulated today. Chemistry in particular, but also other natural sciences and their possibilities, the development of large-scale facilities for laboratory-tested processes and innovations in general must be seen as an opportunity to achieve the transition to sustainable aviation over the next two to three decades. On a positive note, there are currently a large number of projects and a wide range of funding instruments in many countries that are consistently pursuing the path we have been on for several years.

